- Study Says Most Parents Don’t Use Car Seats In Ride Share Vehicles Like Uber
- This 12-Year-Old Boy Is A Sophomore Aerospace Engineering Major!
- Fire Safety Experts Warn Of Hand Sanitizer Danger After A Mom and Kids Escape House Fire
- Recall Alert: Peaches May Be The Cause Of Salmonella Outbreak, 68 People Ill
- Summer Vacation In The Days Of COVID: Tips To Stay Safe
- How To Safely Grocery Shop During The Coronavirus Pandemic
- Michigan Teen With Vape-Related Illness Undergoes Double Lung Transplant
- Teen Kicks Off Anti-Vaping Campaign From Hospital Bed
- Teenager Receives Life Sentence For Strangling Sister To Death Over A Wi-Fi Password
- Toddler Falls To Death From 11th Deck of Cruise Ship
RECALL ALERT: Birth Control Pills Recalled Due To Packaging Error
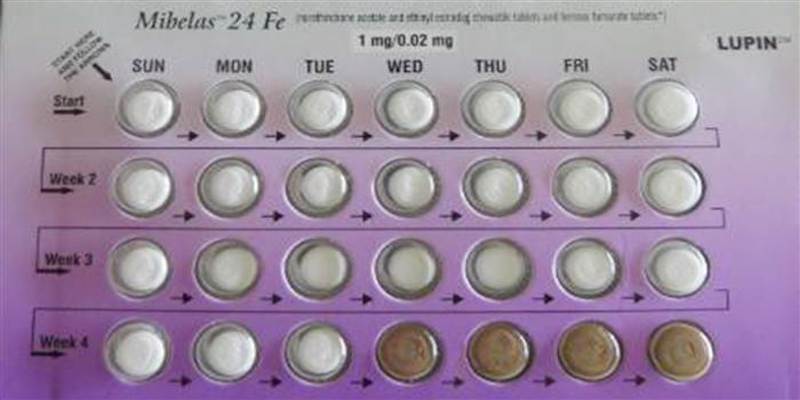

Certain Mibelas 24 Fe birth control pills has been voluntarily recalled by Lupin Pharmaceuticals due to a packaging error that puts placebo pills at the beginning of the pack rather than at the end. This is according to a Food and Drug Administration announcement.
The lot in question is L600518 with an expiration date of May 2018.
Women who have taken the pills in the order they appear in the recalled packs, are at risk of unintended pregnancy. For some, this could have very serious repercussions.
“For patients in whom a pregnancy is contraindicated or in whom concomitant medications may have teratogenic effects, an unintended pregnancy may cause significant adverse maternal or fetal health consequences, including death,” the FDA announcement said.
Dr. Lauren Streicher, an associate professor of obstetrics and gynecology at the Feinberg School of Medicine at Northwestern University and director of the Northwestern Medicine Center for Sexual Medicine and Menopause said the risk of unplanned pregnancy associated with these pills is very real.
“If you take these first four pills thinking that they are the real thing, you may be off the pill for eight days instead of four and that increases the likelihood of inadvertent pregnancy,” Streicher said, adding that it’s not like missing a single pill in the middle or end of a cycle, which does not increase the risk.
If you are already in the middle of your cycle with these pills, but have not had sex, then the risk isn’t elevated, though you will need to use a back-up means of pregnancy prevention, such as condoms, Streicher said.
For those who have already started a pack of the out-of-sequence pills who have had sex, Streicher’s advice is: “Pray. And continue to take the rest of the pack and use back-up contraception for the remainder of the cycle, such as condoms. If they don’t get their period when they finish the pack, do a pregnancy test.”
The FDA recommends that “Consumers with questions regarding this recall can contact Lupin by phone 1-800-399-2561, 8:00 am to 5:00 pm EST, Monday through Friday.”








0 comments